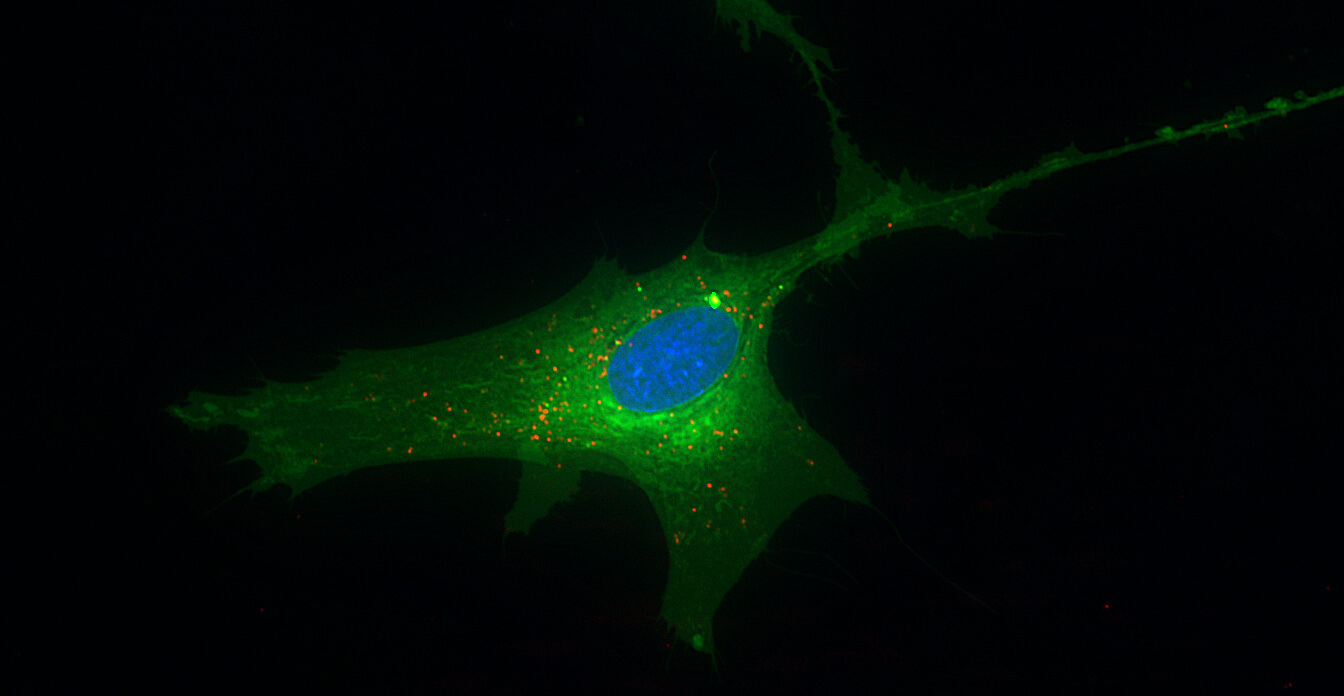
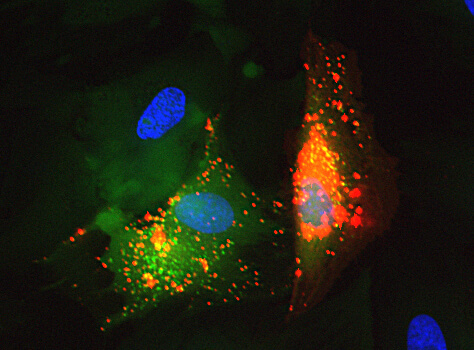
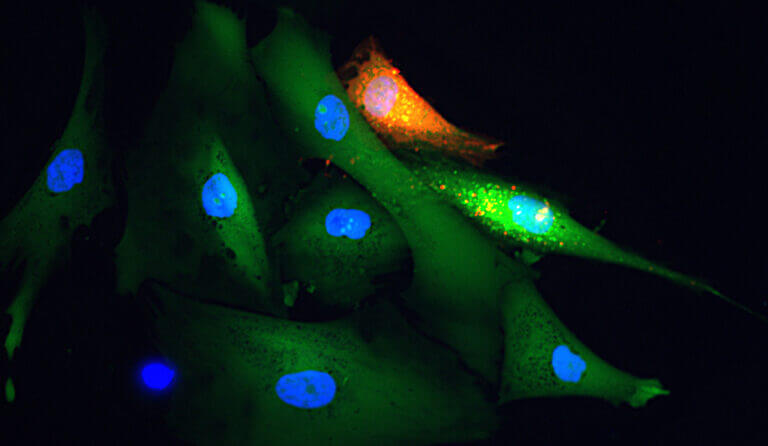
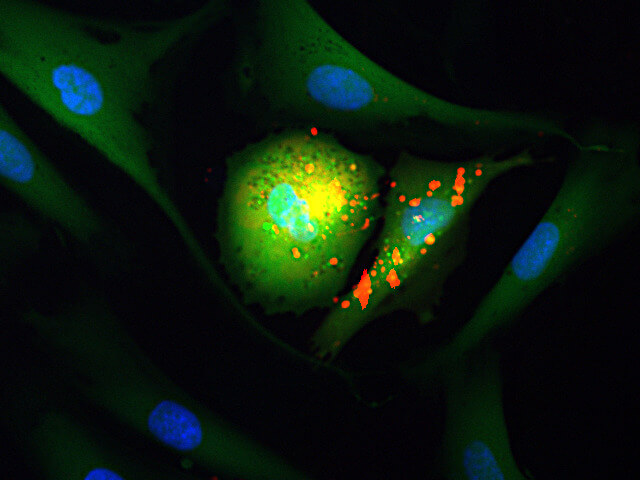
Aegle Therapeutics is a pioneer in regenerative medicine developing extracellular vesicle (EV) therapy to meaningfully improve the lives of those suffering from debilitating rare diseases, including dystrophic epidermolysis bullosa (DEB) and other severe dermatological disorders. Aegle’s platform technology has the potential to treat a wide range of other therapeutic indications.
About Us
Understanding EVs
AGLE-102™
NOW ENROLLING: Phase 1/2 DEB Trial
Our phase 1/2 clinical study involving AGLE-102 in individuals living with Recessive Dystrophic Epidermolysis Bullosa (RDEB) is now open for enrollment. For more information regarding the trial, please read our Informational Brochure or visit ClinicalTrials.gov.
Publications
The effect of mesenchymal stem cells improves the healing of burn wounds: a phase 1 dose-escalation clinical trial. Scars Burn Heal. 2022 Jun 28;8:20595131211070783.
Proteomic analysis of bone marrow-derived mesenchymal stem cell extracellular vesicles from healthy donors: implications for proliferation, angiogenesis, Wnt signaling and the basement membrane. Stem Cell Res Ther 12, 328 (2021).
Dual mechanism of type VII collagen transfer by bone marrow mesenchymal stem cell extracellular vesicles to recessive dystrophic epidermolysis bullosa fibroblasts. Biochimie. 2018 Dec;155:50-58.